PP405 has been making the news lately as a potential revolutionary treatment for the most common form of hair loss, androgenetic alopecia. The holy grail in regenerative medicine for the treatment of androgenetic alopecia would be to find “the key” to that prone hair follicle stem cell and unlock the program to have the follicle revert to producing longer and thicker hair shafts like it did in the past. In the following article, we’ll explore whether PP405 has the potential to meet these expectations.
What is PP405?
PP405 is a topical gel solution that contains a peptide as an active ingredient and helps treat androgenetic alopecia in both men and women. For example, insulin is a peptide that unlocks the ability for cells to absorb glucose. Similarly, the peptide in PP405 may act as the key that stimulates hair follicle stem cells to re-enter the growing phase (anagen), leading to the growth of thicker and longer hair.
PP405 was first discovered by UCLA scientists in 2013, and now it is being developed by Pelage Pharmaceuticals. It is now going through clinical trials to test its efficacy and safety for humans.
You can read the press release by Pelage Pharmaceutical here.
How Does PP405 Work?
PP405 helps dormant hair follicles start growing again by activating the stem cells inside them. This process encourages the follicles to move from the resting phase into the active growth phase, leading to thicker and healthier hair. The expectation is that it can regrow hair follicles even in bald areas by reactivating the stem cells. This is why it is being hyped up so much in the media.
Early research suggests that a 0.05% solution of PP405 applied daily may work on these 3 important points.
- Mitochondrial pyruvate carrier inhibition
PP405 has been shown to inhibit the mitochondrial pyruvate carrier, which is responsible for transporting pyruvate into the mitochondria to generate energy. By slowing this transport, stem cells may shift their metabolism in a way that supports regeneration rather than dormancy. In hair follicle stem cells, this change in energy regulation could help “wake up” follicles that are stuck in the resting (telogen) phase and encourage them to re-enter the growth (anagen) phase. - Increase in Ki67 expression
Ki67 is a protein that scientists use as a marker of cell proliferation. When levels of Ki67 rise, it indicates that cells are actively dividing and multiplying. In the context of hair loss, increased Ki67 expression in follicle stem cells suggests that PP405 is stimulating these cells to renew themselves and produce new hair shafts. This is an important sign that the treatment may directly promote hair regrowth at the cellular level. - Activation of Wnt/β-catenin signaling pathways
The Wnt/β-catenin signaling pathway is one of the most important biological systems involved in hair follicle development and cycling. When activated, it signals dormant follicles to transition back into the anagen (growth) phase. Many hair loss treatments indirectly influence this pathway, but early findings suggest that PP405 may activate it more directly. This could make PP405 especially effective at reawakening miniaturized follicles and supporting the growth of thicker, healthier hair.
Also Read: Creatine and Hair Loss: What You Need to Know?
PP405 Study Results
Phase 2a Trial Findings
In a recent Phase 2a clinical study by Pelage Pharmaceuticals, PP405 was tested on 78 men and women aged 18–55 who were experiencing hair loss.
- 31% of participants achieved at least 20% more hair growth within just 8 weeks of daily application.
- Results were observed in both men and women, so PP405 could potentially be used to treat androgenetic alopecia in both genders.
PP405 vs Current Hair Loss Medications
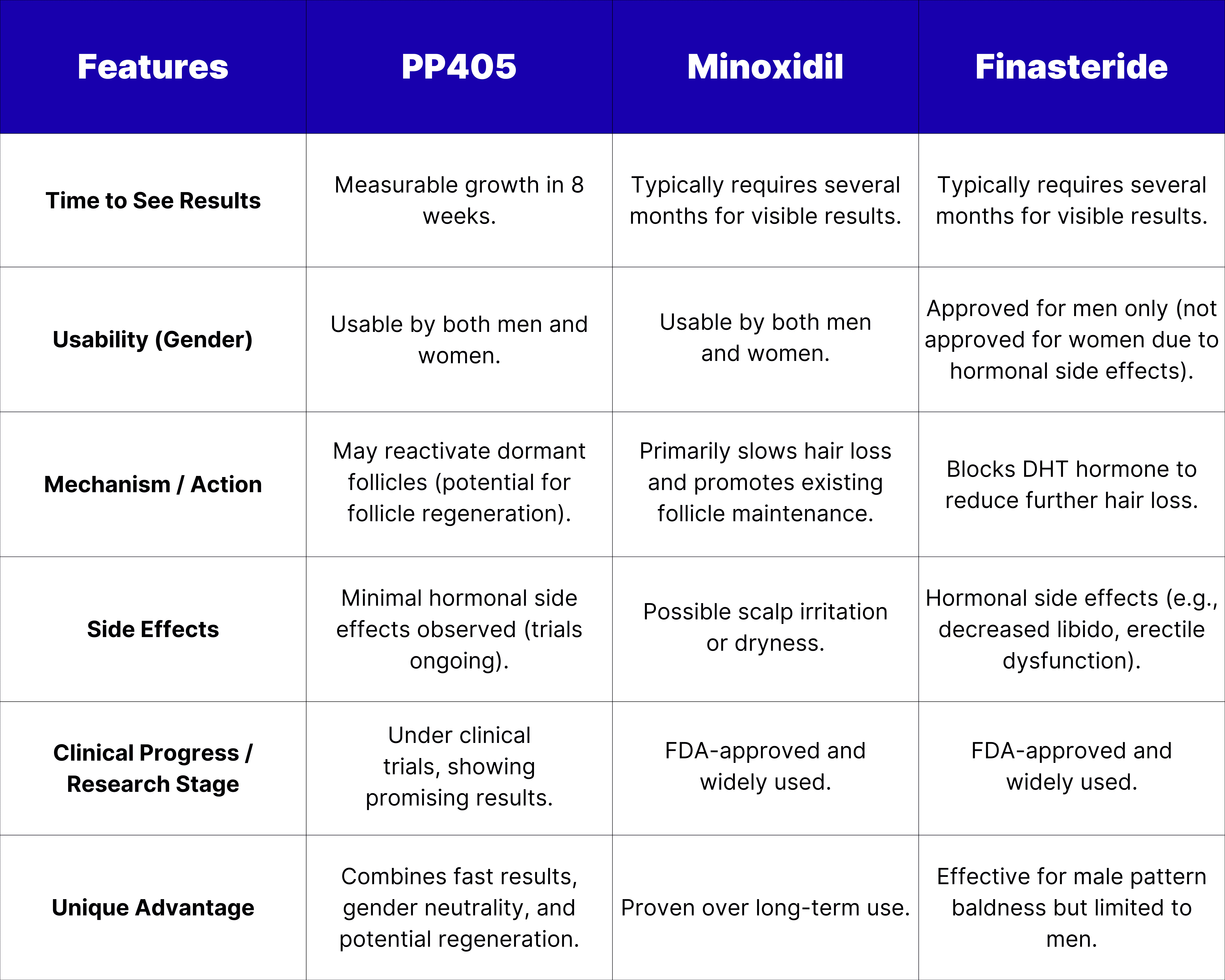
When compared to minoxidil and finasteride, PP405 shows some unique advantages:
- Faster results: PP405 showed measurable growth in 8 weeks, while minoxidil and finasteride typically require months to show some good results.
- Usable by both genders: Finasteride is not approved for women due to hormonal side effects, but PP405 trials showed growth in both men and women. This is quite evident by what the chief medical officer, Qing Yu Christina Weng, MD, said: “We’re excited about that from both being able to have an impact on all genders, but also, you can minimize a lot the hormonal side effects that you see with drug finasteride.” You can read more about her interview here.
- Potential for follicle regeneration: Unlike minoxidil and finasteride, which mainly slow loss or maintain existing hair, PP405 may help reactivate dormant follicles.
If future studies confirm these results, PP405 could represent a major step forward in hair restoration therapy. You can see a more detailed comparison in the table below.
Side Effects of PP405
Because PP405 is still in the early stages of clinical testing, its side effects are not yet fully known. Large-scale and long-term studies will be needed to confirm its safety.
The initial clinical trials are encouraging, but patients should remember that all new medications carry potential risks.
PP405 Before and After Results
At this time, there are no official before-and-after photos released from PP405 trials. The only published data confirms that 24 out of 78 patients achieved a 20% increase in hair growth within 8 weeks.
Any images circulating online claiming to show PP405 results should be viewed with caution until validated by peer-reviewed publications.
Is PP405 Available for Hair Loss Treatment?
No. PP405 is not available for sale. You cannot buy it anywhere as it is still in the clinical trial phase. Even if all the remaining trials go smoothly, it will likely take a long time before approval and availability.
Important warning: If you come across any website selling PP405 or products using its name, it is almost certainly a scam.
Also Read: Taiwan’s New Hair Regrowth Serum: Fact or Fiction
When will PP405 be approved by the FDA?
It’s still in the early stages of the FDA approval process in determining its safety and efficacy. In 2024, PP405 entered phase 2a randomized, placebo-controlled trials, which are assessing the safety, dosing, and efficacy in treating androgenetic alopecia. If it continues to show promise, it will progress to Phase 2b and Phase 3 trials. In the perfect scenario of showing great efficacy and minimal side effects with no glitches in the approval process, it will take approximately 5 years to obtain FDA approval to be available on the market. Currently, the only FDA-approved medications for hair loss are finasteride and minoxidil.
You can see the steps that every product waiting for FDA approval goes through in the image below.

My Opinion
We currently do not have a silver bullet to treat androgenetic alopecia. Finasteride, minoxidil, and hair transplantation surgeries have their limitations; therefore, there is a need for a new revolutionary treatment. Unfortunately, since there is no perfect treatment now, it creates the perfect environment for “snake oil” products, which have been abundant over the last several decades. I believe it is imperative to keep an open, but skeptical, mind to new treatments for hair loss. I think PP405 holds significant promise, but we have to be patient while it progresses through the trials. Again, be aware of the similar “knock-off” products that will sound similar and too good to be true, using creative marketing language. If it sounds too good to be true, it probably is. Let’s keep our fingers crossed for PP405.
Worried About Hair Loss? Let’s Talk.
Get trusted answers from Dr. McAndrews, a board-certified hair restoration physician with over 20 years of experience. We serve Los Angeles through our Pasadena office and offer convenient online consultations that you can reserve from anywhere.

Dr. Paul J. McAndrews is a world-renowned expert in hair loss and hair restoration, and one of only two physicians to have served as President of both the ISHRS and ABHRS. He is a Clinical Professor at USC/LAC Medical Center and an Expert Medical Reviewer for the California Medical Board. Known for his artistic, patient-focused approach, Dr. McAndrews performs one hair transplant per day to ensure exceptional, individualized results.